Chemotherapy For Pancreatic Cancer
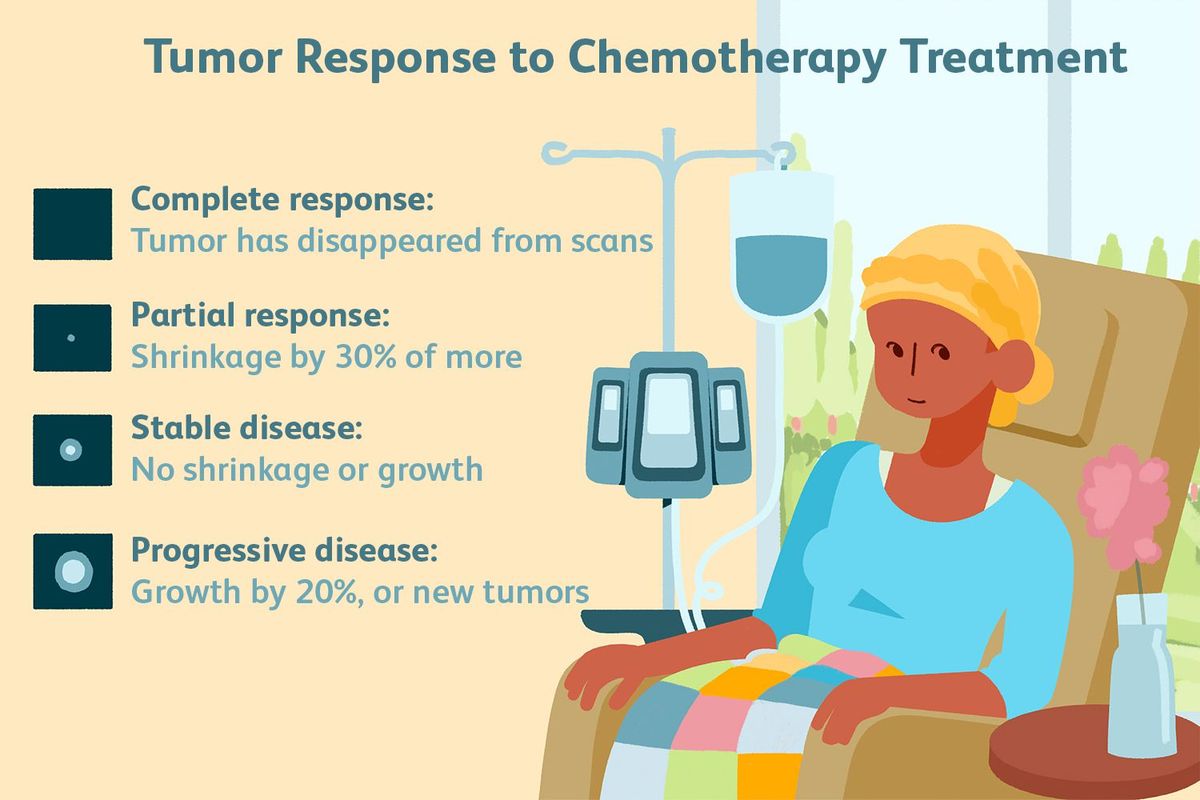
For those with early-stage pancreatic cancer, various options for treatment exist. Treatment options range from Induction chemotherapy, Gemcitabine-based chemoradiotherapy, and PARP inhibitors. Learn about the different options and how they work. The following are common side effects of chemotherapy for pancreatic cancer. Listed below are some of the most common treatments. The best choice for your condition depends on your unique circumstances.
Treatment options for early-stage pancreatic cancer
Several treatments are available for patients with early-stage pancreatic cancer; the most effective is chemotherapy. The cancer is classified by stage and size, and treatments are tailored to the individual patient. Early-stage pancreatic cancer is classified as either locally advanced or metastatic, and its spread to the lymph nodes may require chemoradiation or radiation therapy. Higher radiation doses may be used depending on the size of the tumour.
Patients with borderline resectable pancreatic cancer may receive treatment before surgery, including chemotherapy or targeted therapies. This approach is called neoadjuvant therapy. By shrinking the tumour before surgery, chemoradiation may prevent cancer from returning or spreading to other body parts. It can also improve the patient's quality of life, reduce the risk of recurrence, and prolong survival.
Induction chemotherapy
While induction chemotherapy for pancreatic cancer is often the first option considered, it is not the only one. Other treatments may also be used, such as chemotherapy administered early after diagnosis. In addition to cytotoxic drugs, induction chemotherapy may include radiotherapy, surgery, and radiation therapy. Induction chemotherapy for pancreatic cancer may also have mFOLFIRINOX, a radiotherapy derivative that targets the pancreatic gland.
While induction chemotherapy has many benefits, it is not yet proven to be a lifesaving option. However, it can minimize the toxic effects of radiation therapy. In addition, patients must undergo an extended stay in the hospital, which may not be suitable for everyone. Induction chemotherapy may not be appropriate for all patients, though. In addition, there is no definitive evidence to support the use of this treatment for pancreatic cancer.
Gemcitabine-based chemoradiotherapy
A Phase I/II trial evaluated gemcitabine-based chemoradiotherapy for pancreatic cancer. The treatment consisted of three cycles of 1000 mg/m2 administered intravenously on days one to eight. Then, patients received 830 mg twice daily for the following 21 days. After each cycle, patients were evaluated for toxicity, GI side effects, and hematologic responses.
The LAP07 trial, a multicenter, randomized trial, evaluated the safety and efficacy of gemcitabine-based chemoradiotherapy for pancreatic cancer. The study involved patients with locally advanced pancreatic cancer. Participants were randomized to receive one of the two therapies in a 1:1 ratio, stratified by centre and performance status. The trial coordinating centre was GERCOR in Paris. It was designed to minimize side effects. Patients were randomized in two stages: the first was given induction chemotherapy, while the second was administered gemcitabine-based chemoradiotherapy. The second phase included patients who had progressed from the first step to the second phase.
PARP inhibitors
The potential for PARP inhibitors in treating pancreatic cancer is vast, especially given that the drug targets the BRCA gene, a common genetic mutation in pancreatic cancer. Research on PARPis has focused on cancerous cells that lack BRCA genes, but this principle may also apply to other genetic mutations. With additional studies, a new drug could soon come along that targets PARP1.
Although PARP inhibitors have great potential in pancreatic cancer chemotherapy, there are still numerous hurdles that must be overcome before these drugs are widely used. Developing an improved predictor for PARP inhibitors in pancreatic cancer chemotherapy will be essential. To make a more accurate prediction, it will be necessary to identify suitable candidates and monitor tumour progression over time. Further, the drug may be resistant to some cancers.




